Exportins and NESs
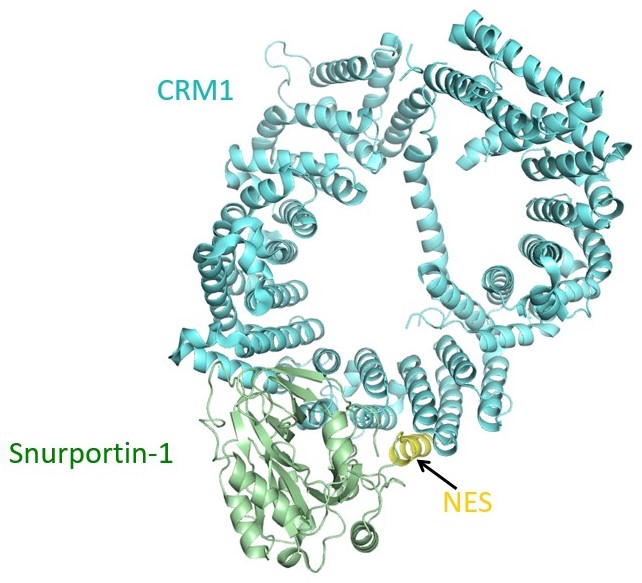
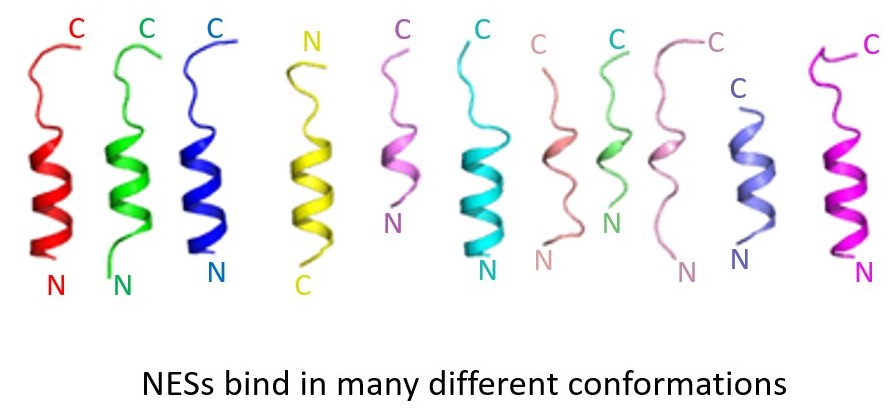
The only known NES is the leucine-rich or classical-NES, which is recognized by the exportin CRM1 or XPO1. In 2009, the Chook Lab reported the crystal structure of CRM1 bound to NES-containing cargo Snurportin 1. This structure showed for the first time how CRM1 recognizes an NES. We revealed requirements for NES recognition, both through crystallography, through compilation/analysis of an NES database named NESdb and through development of an NES predictor named LocNES. Knowledge of CRM1 structure and NES recognition were critical for design of CRM1 inhibitors (KPT-SINE drugs) that are currently in clinical trials for cancers. We revealed unexpectedly different chemical mechanisms for diverse CRM1 inhibitors that were originally thought to act similarly. The differences may explain long-lived clinical toxicity of natural product inhibitor LeptomycinB versus greatly improved tolerance of the new KPT drugs.
